co2 lewis structure
Since it is bonded to only one carbon atom it must form a. In the lewis structure of CO there is a triple bond between the carbon and oxygen atom.
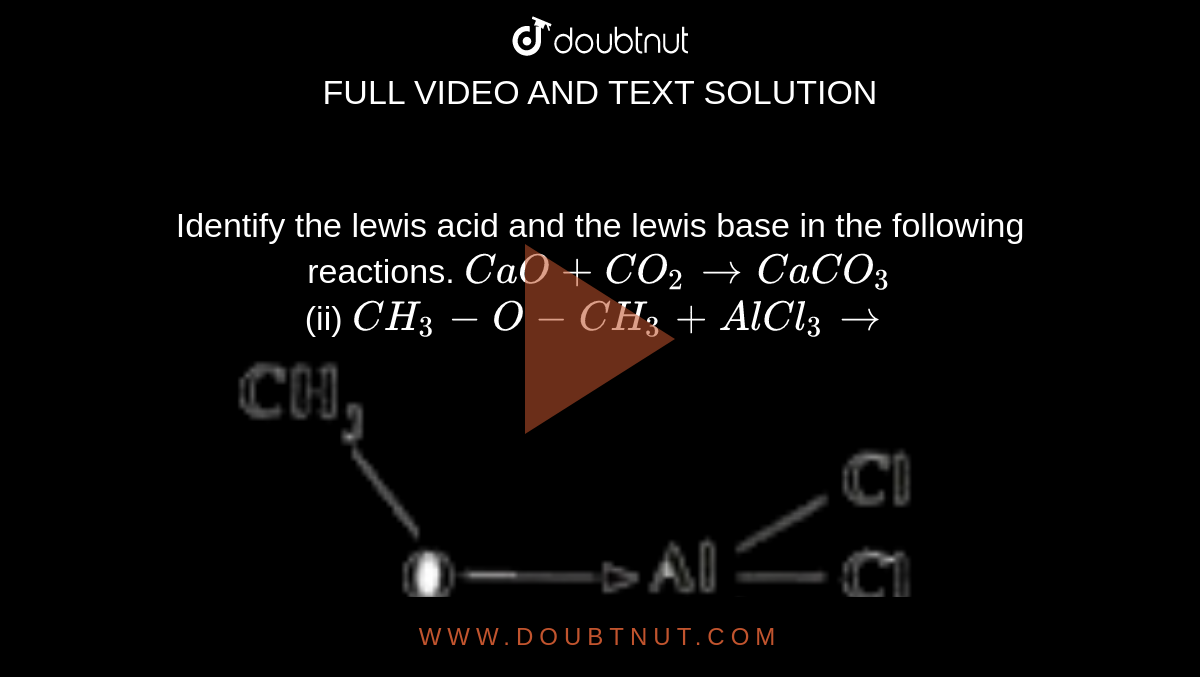 |
| Identify The Lewis Acid And The Lewis Base In The Following Reactions Cao Co2 To Caco3 Ii Ch3 O Ch3 Alcl3 To |
Carbon shares two electrons with each oxygen atom.

. Find out the total number of valence electrons. This is the Lewis structure for CO 2 Carbon Dioxide. The mole fraction refers to the ratio between the. The carbon C atom is kept at the central position and the Oxygen O atom is on either side of.
CO2 Lewis structure is made up of one carbon C atom and two oxygen O atoms. CO2 is made up of two different atoms. The Lewis structure of CO2 is the final stable structure exhibiting no lone pairs and a carbon-oxygen double bond. In the periodic table Oxygen.
The total valence electrons. CO2 lewis structure has a Carbon atom C at the center which is surrounded by two Oxygen atoms O. Determine the total number of electrons in the carbon and oxygen valence shells. Total electron pairs exist in the form of.
In addition to this the contribution of the. This structure is also known as a Lewis dot structure. In the lewis structure of CO 2 there are two double bonds around the carbon atom with two oxygen atoms. 3 rows Lewis structure of CO2 or Carbon Dioxide contains two double bonds between the Carbon.
CO 2 carbon dioxide has one carbon atom and two oxygen atoms. Lewis Structure of CO2 The central atom of this molecule is carbon. Carbon C and Oxygen O so. The CO2 Lewis Structure is where the central carbon atom is the central atom the least electronegative atom with O surrounded by four dots.
There are 2 double bonds between the Carbon atom C and each Oxygen atom O. How to Draw Lewis Structure of CO 2 1. The structure of carbon dioxide contains one carbon atom and 2 oxygen atoms. In the carbon dioxide Lewis structure the carbon atom is in the central position as it is the least electronegative atom in the molecule.
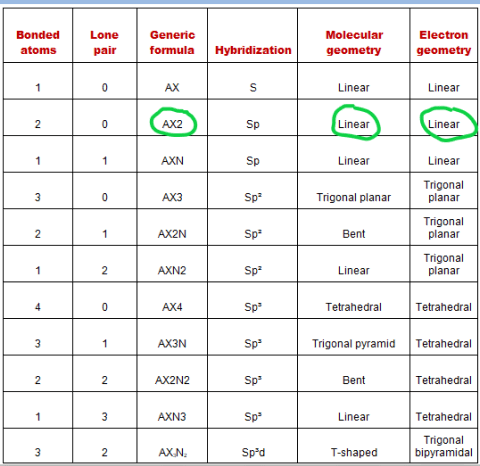
The total valence electron is 16 for drawing CO2 Lewis structure and it shows molecular geometry is linear. Oxygen contains 6 valence electrons which form 2 lone pairs. Lewis Structure of Carbon Monoxide CO In rm CO oxygen belongs to group 16 of the Periodic Table and carbon belongs to group 14 of the Periodic Table. The Lewis Dot Structure for carbon dioxide can be represented like this.
Solubility for the CO2 as represented by the lewis structure is measured based on the composition in terms of the mole fraction. The Lewis structure of carbon monoxide CO has a triple bond formation where one is strong sigma and the other two are weak pi bonds. What is a Lewis Dot Structure and what do the symbols in carbon dioxides. The valence electrons of carbon are four and oxygen 6 since 2 atoms of oxygen are present the total.
The molar mass of CO2 is 44009 gmol and density is 1562 kgm3. Steps to be followed for drawing CO2 Lewis structure 1. But what exactly does this mean. This means that we would have.
CO Lewis Structure CO carbon monoxide has one carbon atom and one oxygen atom. Explain the CO2 Lewis structure in simple words. What is the Lewis structure of CO2.
 |
| Solved For The Co2molecule A Draw The Lewis Structure B Identify How Course Hero |
 |
| Co2 Lewis Structure Drawing Method Of Co2 Lewis Structure Molecular Geometry Of Co2 Polarity And Hybridisation In Co2 Molecule With Faqs |
 |
| Co2 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist |
 |
| Co2 Lewis Structure Easy Hard Science |
 |
| Co2 Lewis Structure Molecular Geometry Bond Angle Polar Or Nonpolar |
Posting Komentar untuk "co2 lewis structure"